product portfolio
Our product portfolio is built on botanical compounds that have the potential to address worldwide unmet medical needs for a variety of gastrointestinal indications.

potential indications
Crofelemer is a novel, first-in-class inhibitory modulator of two secretory chloride ion channels in the gastrointestinal tract. As an antisecretory, antidiarrheal drug, it has a normalizing effect on the electrolyte and fluid balance in the gut. This mechanism of action has the potential to benefit multiple gastrointestinal disorders. Crofelemer has been studied in more than 3,000 patients to date, for a variety of potential indications. Crofelemer is FDA-approved for symptomatic relief of noninfectious diarrhea in adults living with HIV/AIDS who are on antiretroviral therapy (ART).
Crofelemer (delayed-
release tablets): Napo
Pharmaceuticals–
sponsored trials

Crofelemer (powder for
oral solution): Napo
Pharmaceuticals-
sponsored trials
NP-300 (tablets):
Napo Pharmaceuticals-
sponsored trial
| Indications Evaluated | Development Stage | |||||||
| Pre-IND | IND / CTA | Phase 1 | Phase 2 | Phase 3 | Commercialized (US) |
Geographic focus of clinical studies |
||
| |
||||||||
| Noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy | ||||||||
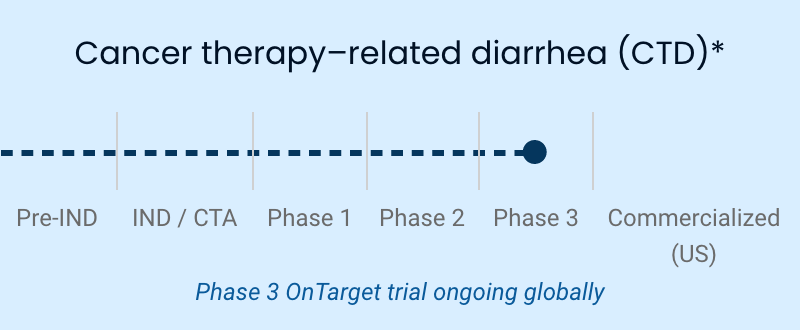
| Cancer therapy-related diarrhea (CTD) | Phase 3 OnTarget trial ongoing globally |
|||||||
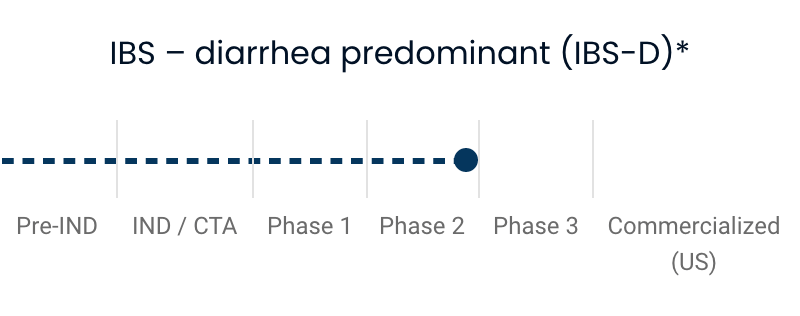
| IBS—diarrhea predominant (IBS-D) | |
|||||||
| |
||||||||
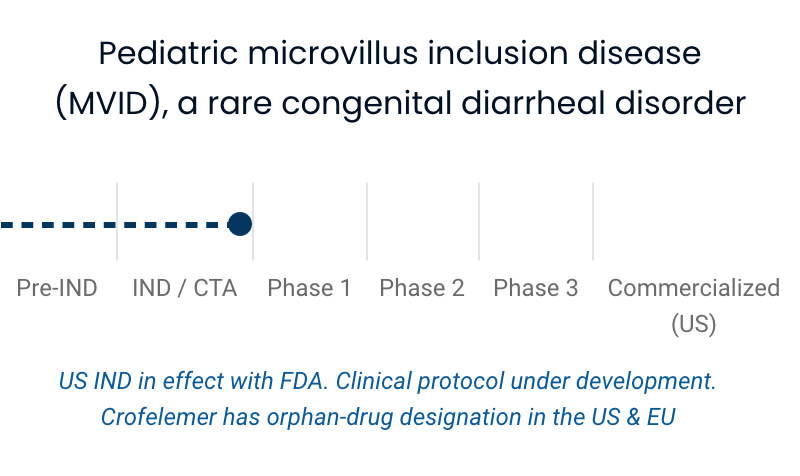
| Pediatric microvillus inclusion disease (MVID), a rare congenital diarrheal disorder | US IND in effect with FDA. Clinical protocol under development. Crofelemer has orphan-drug designation in the US & EU |
US | ||||||
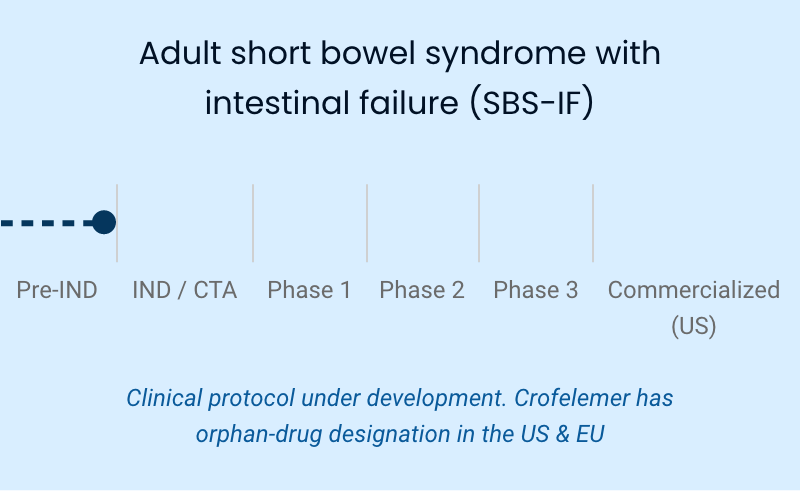
| Adult short bowel syndrome with intestinal failure (SBS-IF) | Clinical protocol under development. Crofelemer has orphan-drug designation in the US & EU |
US &: EU | ||||||
| |
||||||||
| Symptomatic relief and treatment of diarrhea from infectious pathogens | |
US IND in effect with FDA | ||||||
ongoing investigator-initiated trials
NCT03898856: Click here to view trial overview
*Geographic focus of clinical studies: US
ongoing investigator-initiated trials
NCT03898856:
Click here to view trial overview
indication
Mytesi® (crofelemer) is an anti-diarrheal drug indicated for symptomatic relief of non-infectious diarrhea in adults living with HIV/AIDS who are on antiretroviral therapy (ART).
important safety information
Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (6%), bronchitis (4%), cough (4%), flatulence (3%), and increased bilirubin (3%).
NAP-G-0010

